Pipeline
Dry eye diseaseWhen it comes to dry eye disease, it is generally thought of as having early-stage symptoms that can be alleviated with artificial tears. However, as the average human lifespan increases, the number of patients suffering from severe dry eye disease is rapidly increasing, and in severe cases, it is one of terrifying diseases that not only causes corneal inflammation and even blindness.
However, the treatment of the disease has been heavily dependent on two drugs with a mechanism to simply suppress inflammation: Allergan's Restasis and Bausch and Lomb's Xiidra.
A wide therapeutic rangeOur AVI-4015, a DDR1 inhibitor, unlike existing drugs, not only reduces corneal inflammation but also treats corneal damage by inflammation. Accordingly, AVI-4015 has the effect of protecting both corneal epithelial cells and goblet cells that produce tears.
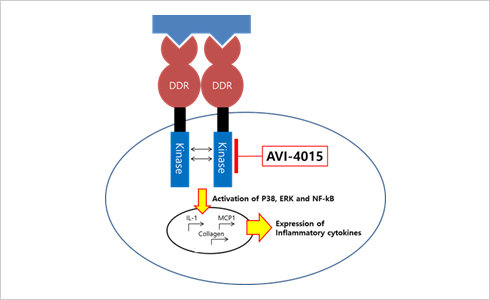
Rapid progress of clinical trialsIn particular, our AVI-4015 was developed as a dry eye disease treatment using an existing FDA-approved drug using a drug repositioning approach, so a lot of clinical data has been accumulated and the supply of the ingredients is smooth.. Therefore, AVI-4015 is expected that clinical trials and commercialization will proceed at a much faster pace than other drugs.